Resources
Package inserts

The T-SPOT.TB test package insert
Download
T-Cell Select reagent package insert
Download Now
T-Cell Xtend® reagent package insert
DownloadBlood draw bulletin

The T-SPOT.TB test specimen requirements
DownloadResults interpretation
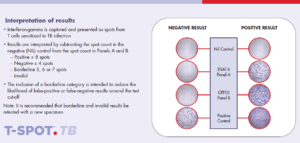
The T-SPOT.TB test results interpretation
VIEWAdditional information

The T-SPOT.TB test 1 million ways to find the truth brochure
Download
The T-SPOT.TB test brochure
Download
Science behind the T-SPOT.TB test
Download
The T-SPOT.TB test frequently asked questions
Download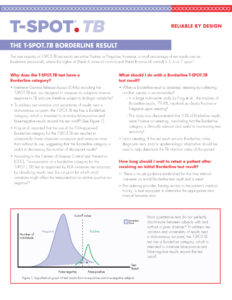
The T-SPOT.TB test borderline result
Download